Introduction: Induction regimens utilising cyclophosphamide, bortezomib and dexamethasone (CyBorD) prior to autologous stem cell transplant are utilised worldwide where access to a combination immunomodulatory agent/proteasome inhibitor approach is limited due to financial or regulatory constraints. Protocols utilising weekly subcutaneous bortezomib are favoured in order to reduce toxicity, but there is a lack of published data regarding response rates and survival using this approach.
Methods: We identified all patients treated between August 2015 and August 2019 at St Vincent's Hospital and January 2012 and August 2019 at Liverpool Hospital in Sydney, Australia who were prescribed four 28 day cycles of CyBorD (subcutaneous bortezomib 1.3mg/m2, cyclophosphamide 300mg/m2 (or 500mg) and dexamethasone 40mg (day 1, 8, 15 and 22) for newly diagnosed MM with an intent to proceed to ASCT.
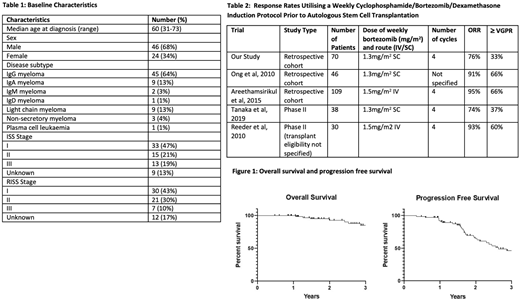
Results: The baseline patient and disease characteristics of the 70 eligible patients are listed in Table 1. Most patients received therapy as planned (62/70) and all but one proceeded to ASCT. Seven patients had additional therapy after 4 cycles of CyBorD due to inadequate response.
Best response to CyBorD induction included stable disease (SD) in 16 (23%) partial response (PR) in 30 (43%), and a VGPR or better in 24 (33%) (2 complete response (CR)). No patient had progressive disease.
All but one patient had successful stem cell mobilisation. The median number of collection days was 2 (range 1-4) and median number of stem cells collected was 10.4 x 106/kg. 6 patients required plerixafor. The one patient who could not be mobilised received lenalidomide prior to mobilisation.
Best response post ASCT (n = 69) prior to unplanned therapy included SD in 2 (3%), PR in 25 (35%), ≥ VGPR in 41 (59%) (4 patients with CR) and undetermined in 1. Of those who had SD following induction, 4 achieved a ≥ VGPR following ASCT, 9 had a PR, 2 had SD and 1 did not undergo ASCT. Five patients underwent a second ASCT due to inadequate response. 37 patients received maintenance.
After a median of 3 years follow-up, 12 patients (17%) had died (10 from progressive disease) and 28 (40%) had progressed. Overall survival at 1 year was 100% and 3 years was 85% (95% CI 71-93%) (Figure 1). Progression free survival at 1 year was 92% (95% CI 88-97%) and 3 years was 46% (95% CI 31-60%) (Figure 1). There were no deaths during induction and no documented bortezomib dose reductions. 16 of 70 patients had unplanned hospital admissions during induction. There was no transplant related mortality.
Conclusions: To our knowledge, this is the largest study evaluating use of a weekly induction protocol of subcutcutaneous bortezomib, oral cyclosphosphamide and oral dexamethasone in transplant eligible MM. It shows comparable response rates and survival outcomes to other retrospective studies and documented toxicity was low. This induction protocol remains standard of care in many countries, in particular where financial constraints limit use of combined IMID/PI induction and this study confirms the efficacy and tolerability of this commonly used protocol.
Ling:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene BMS: Membership on an entity's Board of Directors or advisory committees; Specialised Therapeutics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal